Cryopreservation is an indispensable method that underpins modern biological research and biomanufacturing. By halting biological activity through ultra-low temperature storage, cryopreservation ensures the preservation of cellular integrity, enabling long-term storage without compromising viability or functionality. This process is more than just freezing cells; it is a precise science that demands meticulous preparation, careful selection of cryoprotectants, and optimised thawing protocols. Each phase—from initial preparation to recovery—can significantly impact cell survival rates and experimental reproducibility, making adherence to best practices critical.
In an era where cell-based therapies, vaccine production, and advanced biotechnologies dominate the scientific landscape, the importance of cryopreservation has never been greater. This guide delves into the intricacies of cryopreservation and recovery, providing a comprehensive overview of the methods, challenges, and innovative solutions that define this essential process. Whether you aim to enhance your current protocols or explore the latest advancements, this resource is designed to support your pursuit of excellence in cell culture storage.

Source: cen.acs.org
The Science Behind Cryopreservation
Cryopreservation operates on the fundamental principle of halting biological processes by reducing temperature to sub-zero levels. At these ultra-low temperatures, cellular metabolism and enzymatic activity come to a near standstill, effectively preserving cells in their current state. However, this intricate process relies on carefully managed steps to prevent cellular damage and ensure high post-thaw viability.
How Cryopreservation Works
The process is designed to protect cells from physical and chemical stresses induced by freezing. Key challenges include:
- Ice Crystal Formation: Without protective measures, ice crystals can physically disrupt cell membranes, leading to irreversible damage.
- Osmotic Stress: The freezing process alters the balance of intracellular and extracellular solutes, risking dehydration or rupture.
The Role of Cryoprotectants
Cryoprotectants are essential to minimising damage during freezing. These compounds protect cells by:
- Lowering Freezing Point: Preventing rapid ice crystal formation.
- Reducing Osmotic Imbalances: Stabilizing intracellular and extracellular solute concentrations.
Common cryoprotectants include:
- Dimethyl Sulfoxide (DMSO): Widely used at 5–10% concentrations due to its efficiency in penetrating cell membranes.
- Glycerol: Preferred for certain cell types, such as bacterial cultures, due to its lower toxicity.
The Critical Cooling Rate
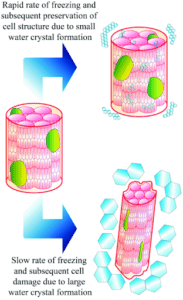
The success of cryopreservation hinges on achieving an optimal cooling rate—generally around -1°C per minute. Cooling too quickly risks intracellular ice formation, while slower rates can lead to osmotic imbalance. Controlled-rate freezers and isopropanol-containing freezing containers are commonly used to maintain this delicate balance.
Source: geeksforgeeks
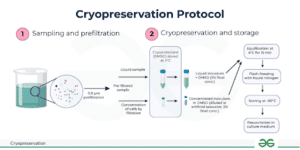
Preparing Cells for Cryopreservation
Preparing cells for cryopreservation is a meticulous process that involves several key steps, each crucial for ensuring cell viability and functionality. Here’s how to do it right:
Step 1: Assessing Cell Health
Healthy cells are the foundation of successful cryopreservation.
- Growth Phase: Cells should be in the logarithmic growth phase, ensuring they are metabolically active and robust.
- Viability: Only use cultures with a viability rate of over 90% to maximize survival during freezing and recovery.
Step 2: Choosing the Right Cryoprotectant
Cryoprotectants safeguard cells by preventing ice crystal formation and osmotic damage.
- DMSO: The gold standard for most cell types, used at 5–10% concentrations.
- Glycerol: Ideal for microbial cultures and some specialized cell lines.
- Serum-Free Alternatives: Emerging solutions for xeno-free applications.
| Cryoprotectant | Advantages | Applications |
| DMSO | Highly effective, membrane-permeable | Mammalian and primary cells |
| Glycerol | Less toxic, cost-effective | Microbial and fungal cultures |
Step 3: Preparing the Freezing Medium
The freezing medium acts as a protective shield during the freezing process:
- Serum-Supplemented Media: Combines cryoprotectants with FBS or BSA for added cellular support.
- Serum-Free Media: Ideal for chemically defined workflows, offering consistency and compatibility.
Step 4: Optimizing Cryovial Preparation
- Sterilise and label cryovials with details like cell type, passage number, and freezing date.
- Fill each vial with 1–1.5 mL of freezing medium to balance freezing efficiency and recovery needs.
Pro TipConduct a small-scale test freeze to assess viability and refine conditions before freezing a full batch. |
The Freezing Process: Precision in Action
Cryopreservation hinges on achieving the perfect freezing process, where temperature reduction is meticulously controlled to protect cellular integrity. Each step is a balancing act to minimise damage and maximize post-thaw viability.
Controlled Rate Freezing
Slow and steady wins the race when it comes to freezing cells. The optimal cooling rate, typically around -1°C to -3°C per minute, allows water to leave the cells gradually, preventing intracellular ice crystal formation. Rapid cooling, on the other hand, risks damaging cell membranes, while overly slow freezing can lead to osmotic stress.
| Freezing Methods | Advantages | Challenges |
| Controlled-Rate Freezers | High precision and consistency | Expensive initial investment |
| Isopropanol-Freezing Units | Affordable and easy to use | Limited to slower cooling rates |
Freezing Medium: A Cellular Safety Net
The freezing medium plays a pivotal role during this phase. It is carefully composed to provide an ideal environment for cells as they transition to ultra-low temperatures.
- Cryoprotectants: Protect against ice crystal damage.
- Supplemental Components: Add FBS, BSA, or serum-free alternatives for added stability.
Cryovial Storage in Liquid Nitrogen
Once frozen, cryovials are transferred to liquid nitrogen storage units. Here’s how to do it right:
- Temperature Management: Store in vapour-phase liquid nitrogen (-150°C to -196°C) to avoid risks of contamination.
- Labelling: Use cryo-safe labels and record critical details for easy retrieval.
Pro Tip for StorageAlways use backup inventory systems to minimize the risk of losing valuable samples due to labelling errors or equipment malfunctions. |
Source: Dang, Davidson & Bastarrachea, Luis & Martini, Silvana & Matarneh, Sulaiman. (2021). Crystallization Behavior and Quality of Frozen Meat. Foods. 10. 2707. 10.3390/foods10112707.
Thawing and Recovery: Revitalizing Your Cells
Thawing cryopreserved cells is a critical phase that requires precision and care. The goal is to revive the cells efficiently while minimizing stress and maximizing viability. Here’s how to master the thawing and recovery process.
Embracing Rapid Thawing
Speed is of the essence when thawing cryopreserved cells. Rapid thawing minimizes the formation of ice crystals, which can damage cell membranes.
- The Process: Remove the cryovial from liquid nitrogen storage and immediately place it in a 37°C water bath. Gently swirl the vial continuously.
- Timing: Aim to thaw the contents within 1 to 2 minutes. Thawing too slowly increases the risk of ice crystal formation, while too rapid can cause thermal shock.
Gentle Removal of Cryoprotectants
Post-thaw, it’s crucial to eliminate cryoprotectants like DMSO, which can be toxic to cells at room temperature.
- Dilution: Transfer the thawed cell suspension carefully into a centrifuge tube containing 9 mL of pre-warmed complete growth medium. This tenfold dilution reduces the concentration of DMSO rapidly.
- Centrifugation: Spin the cells at 200 × g for 5 minutes to pellet them gently.
- Resuspension: Remove the supernatant carefully and resuspend the cell pellet in a fresh, pre-warmed growth medium appropriate for the cell type.
Optimizing Post-Thaw Culture Conditions
After resuspension, cells need an ideal environment to recover and proliferate.
- Seeding Density: Plate the cells at a slightly higher density than usual to promote cell-to-cell interactions, which aid recovery.
- Incubation: Place the culture flask or dish in a humidified incubator set to the appropriate temperature and CO₂ levels for your cells (e.g., 37°C with 5% CO₂ for mammalian cells).
- Monitoring: Over the next 24 to 48 hours, monitor the cells for adherence (if they are adherent cells), morphology, and signs of contamination.
Overcoming Common Recovery Challenges
Despite careful handling, you may encounter some issues:
Low Viability Post-Thaw
- Cause: Prolonged exposure to cryoprotectants or improper thawing techniques.
- Solution: Ensure rapid thawing and immediate dilution of cryoprotectants. Consider using specialized recovery media enriched with growth factors.
Clumping of Cells
- Cause: DNA release from damaged cells can cause remaining viable cells to stick together.
- Solution: Add DNase I to the resuspension medium to degrade free DNA, and gently pipette the suspension to separate clumps.
Enhancing Recovery with Supplements
Incorporating recovery supplements can boost cell survival:
- Antioxidants: Compounds like glutathione can reduce oxidative stress during recovery.
- Growth Factors: Adding specific cytokines or growth factors tailored to your cell type can promote proliferation.
A Success Story: Reviving Delicate Stem Cells
When working with delicate human pluripotent stem cells (hPSCs), researchers often face low recovery rates. By implementing a rapid thaw protocol combined with a specialized, serum-free recovery medium enriched with antioxidants and growth factors, one team improved their post-thaw viability from 50% to over 85%. This adjustment not only saved time and resources but also enhanced the consistency of their experimental results.
Common Challenges in Cryopreservation and Recovery
Even with the best protocols, challenges can arise during cryopreservation and recovery. Here’s a quick guide to identifying and overcoming these obstacles:
✅ Ice Crystal Formation: The Cellular Villain
- The Problem: Ice crystals can rupture cell membranes, causing irreversible damage.
- The Fix: Use cryoprotectants like DMSO or glycerol and maintain a controlled cooling rate of -1°C per minute to prevent intracellular freezing.
✅ Cryoprotectant Toxicity: A Delicate Balance
- The Problem: Prolonged exposure to cryoprotectants can harm cells.
- The Fix: Minimize cryoprotectant exposure by diluting and removing them promptly during the recovery process.
✅ Loss of Viability Post-Thaw
- The Problem: Some cells fail to survive thawing despite careful freezing.
- The Fix: Thaw cells rapidly in a 37°C water bath and immediately resuspend them in a nutrient-rich medium to support recovery.
✅ Contamination: The Silent Threat
- The Problem: Bacterial, fungal, or mycoplasma contamination can jeopardize cell integrity.
- The Fix: Maintain strict aseptic conditions during freezing and thawing, and regularly test cell lines for sterility.
✅ Genetic Drift and Cellular Alterations
- The Problem: Long-term storage or repeated freeze-thaw cycles can lead to genetic drift.
- The Fix: Limit freeze-thaw cycles and document detailed records for each batch to ensure consistency.
| Pro Tip for Troubleshooting
Keep a meticulous record of your freezing and thawing processes. Patterns in these logs can help you refine and improve your protocols. |
Conclusion
Cryopreservation and recovery are more than just essential techniques—they are the lifeline for preserving the future of research and biomanufacturing. By carefully selecting cryoprotectants, mastering freezing protocols, and optimizing recovery methods, researchers can ensure the longevity and functionality of their precious cell cultures.
As science continues to evolve, innovations in cryopreservation—like serum-free media and automated freezing systems—are paving the way for greater efficiency and precision. By staying informed and adhering to best practices, you can overcome common challenges, protect your cells from damage, and achieve consistent, reliable results.
Whether you’re working on groundbreaking cell-based therapies or simply maintaining a robust cell line inventory, the principles of cryopreservation are your strongest ally. With the right approach, you’re not just storing cells—you’re preserving potential, unlocking opportunities for discoveries that can transform the world.